An Analysis of the Effects of Caffeine on the Heart Rate of Ghost Shrimp
by Karly Garnto and Austin Satterfield
Caffeine is an extremely common stimulant used in modern society to help humans stay awake. Caffeine is becoming a more common pollutant in freshwater habitats, so its effects on small aquatic invertebrates may be important for the future of these environments. This study examines the effects of caffeine on the heart rate of Palaemonetes paludosus, more commonly called the ghost shrimp. It was hypothesized that the effect of caffeine would be similar to that in humans, with an increase in heart rate correlated with an increase in caffeine concentration. The ghost shrimp heart rate was monitored under a microscope in five different, increasing caffeine solutions. Contrary to the hypothesis, caffeine did not increase the heart rate; however, analysis of the data showed a slight decrease in heart rate over increasing caffeine concentration. Further research indicates that the absence of adenosine receptors in the ghost shrimp may be the reason behind the miscorrelation with the results of caffeine in humans.
Introduction:
Eighty percent of the world’s population consumes caffeine on a daily basis, making it the most common psychoactive drug in the world [1]. Most people enjoy drinking this substance in the form of coffee, soda, or tea and find it necessary to provide them with the alertness needed to complete daily tasks. No detrimentally negative effects have been found in adults who consume large amounts of caffeine, and it has actually been suggested that the drug may have some positive effects [2]. However, as humans, it is important to know the effects that consuming large amounts of caffeine have on the body, due to its vast consumption by a large portion of society.
Caffeine is a methylxanthine that causes stimulating effects in humans by binding competitively to the adenosine receptors in the brain. Adenosine is the naturally produced chemical messenger in humans that binds to the adenosine receptor, enabling the body to slow down and prepare for sleep [3]. When caffeine binds to the receptor instead, it triggers hormones such as epinephrine and norepinephrine to be released into the bloodstream, so the pituitary gland does not receive the chemical signal to slow down bodily activities such as heart rate (Smits et al. 1987).
It may keep humans awake, but what are caffeine’s effects on other organisms? Recent studies involving the increasing concentration of organic waste compounds (OWCs) have found that caffeine is actually a rising threat to freshwater rivers and lakes. Even though caffeine is being released into aquatic environments in low doses, it could still have a profound effect on freshwater organisms [4]. This information has sparked interest in the effects of high caffeine levels on the heart rates of organisms integral to these aquatic ecosystems. OWCs such as caffeine enter freshwater ecosystems in the same way as other, more common human waste, through urban sewage systems or storms, which produce elevated water levels and an overflow of untreated wastewater into the surrounding lakes of urban and suburban areas (Phillips and Chalmers 2009). In a study done by Moore et al. (2008), caffeine was not shown to be an immediate danger to freshwater organisms given the small median concentration of 0.1 μg/L in freshwater lakes and rivers. However, another study has shown wastewater influent sample levels of caffeine in South Carolina freshwater systems in excess of 83 μg/L, which is substantially higher than shown by previous research (Garcia et al. 2014). Despite the observation that human disposal of caffeine into local waterways is increasing, the number of studies on caffeine’s effects on other organisms is still surprisingly small.
One study on the adaptability of small aquatic invertebrates to different, more naturally occurring environmental stimuli, such as decreased salinity and varied temperatures, was conducted by Defur and Mangum (1979). This study showed that while the responses to decreased salinity and other stimuli varied, the results suggested that arthropods, in this case the Atlantic horseshoe crab (Limulus polyphemus), crustaceans such as the blue crab (Callinectes sapidus), and the purple shore crab (Hemigrapsus nudus), have more variable heart rates than other invertebrates like mollusks and annelids. The number of cardiac arrests that took place during their experiments was also documented and found to be less frequent in arthropods even though their heart rates have the most variability of the tested aquatic organisms [5]. Adaptability and a knowledge gap surrounding the responses of the ghost shrimp (Palaemonetes paludosus) to environmental stressors made it an ideal freshwater invertebrate to study.
Ghost shrimp are typically about 2 inches long and are transparent, so their inner organs are easily observed. Because of this organism’s impressive ability to withstand more environmental stress than other small aquatic organisms, this study proposed to investigate the effects of increasing concentrations of caffeine on ghost shrimp’s heart rates. Due to the fact that ghost shrimp are not widely studied like some of their other arthropod counterparts, such as the crabs observed by Defur and Mangum [5], the results of this study may provide further insight on the biochemical connections between humans or other vertebrates and invertebrates. We also seek to establish a baseline for further study of the ghost shrimp. We hypothesize that the heart rate of the shrimp will increase as the amount of caffeine (a stimulant for many organisms) in their surrounding environment increases.
Materials and Methods:
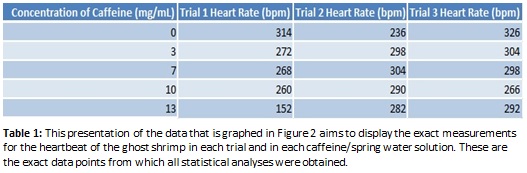
The first step to this experiment involved finding a consistent way to obtain different levels of caffeine for the shrimp to absorb without having the added ingredients, such as sugar and starches, present in the substances that humans typically use to obtain caffeine. In order to accomplish this, three 50 mL dilutions were made from a 13 mg/mL solution of caffeine provided by the lab. Spring water was used to dilute the caffeine solution instead of tap water to prevent unknown chemicals and particles from getting into the solutions. Our five caffeine solutions contained 13 mg/mL (highest amount), 10 mg/mL, 7 mg/mL, 3 mg/mL and 0mg/mL (50 mL of just spring water) which acted as our control for the stress of being moved into experimental containers. Because ghost shrimp are transparent invertebrates, it is not difficult to see the different organs in their bodies, especially when placed under a microscope. The shrimp were constrained for microscopic observation using “foamy dishes” that were created to serve the same purpose as the foamy slides often used in lab to view live microscopic organisms. We placed layers of foam into petri dishes after cutting out a square hole in the center of the foam to keep the specimen from swimming around in the dish. One dish was created for each of the 5 caffeine and spring water solutions and in each dish, one shrimp was examined at a time. For each different solution, the one being used was poured into the “foamy dish” using a plastic pipette and the shrimp was then fished out of its pond water habitat and placed in the center of the dish. Under the microscope, the shrimp’s heart can be found in the upper part of its torso, under its eyes. The heart is transparent so it is difficult to see the actual organ but the beating motion is visible when you look closely between the other organs surrounding the heart. The shrimp were examined using the video screen microscope available in lab and manual counters were used to count the heartbeat. In each solution we performed three, 30 second trials where two people counted, one person kept time and the fourth person kept the microscope in focus. For each trial, the numbers on the two counters were averaged, and then that average was multiplied by two to obtain the beats per minute.
Results:
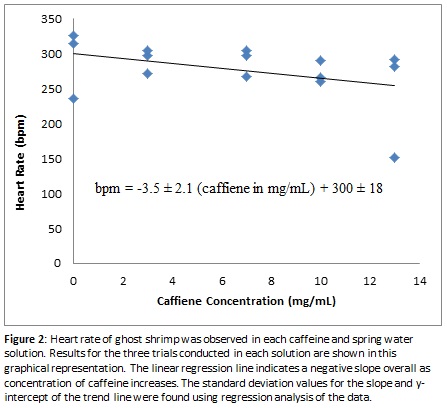
The results shown in Figure 2 indicate with certainty that in higher caffeine levels a ghost shrimp’s heart rate does not increase as we hypothesized, but may in fact decrease slightly instead. To verify this conclusion, the calculated standard error of the slope representing the trend in bpm in the increasing caffeine solutions shown in Figure 2, a negative slope maintains prominence to indicate there is strong statistical evidence that heart rate decreases with increasing levels of caffeine in solution. Considering the heart rate in each trial varied slightly and the heart rate between each different caffeine concentration did not vary as greatly as expected, human error needs to be taken into account when analyzing the effects of increased caffeine levels.
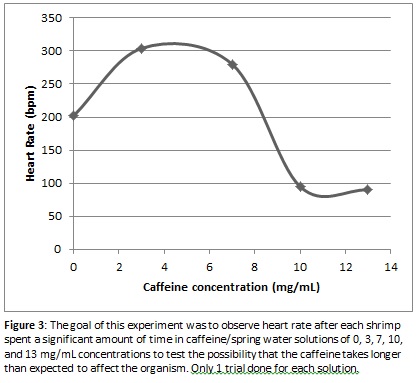
Since the movement from their natural environment to a purely caffeine and spring water solution could have aggravated the shrimp and caused a faster-than-normal heart rate, we decided to conduct one set of extra trials to observe whether keeping the shrimp in the caffeine solution for an increased amount of time would give the substance more time to take effect and would give the shrimp a chance to calm down. This side experiment involved one trial each in the 0, 3, 7, 10 and 13 mg/mL solutions. The shrimp specimen was kept in the foamy dish containing the solution for an extra 10 minutes after the initial examination, then data was collected again using the same method that was used in the first experiment. As seen in Figure 3, the results of this experiment showed a much sharper decline in heart rate as the caffeine level increased after the first initial increase in heart rate from 0 mg/mL to 3 mg/mL. This initial jump could be due to error since only one trial was conducted, or it could indicate that shrimp heart rate does increase initially but tapers off and begins decreasing when the concentration gets above a certain concentration of caffeine.
Discussion:
The downward trends of both Figure 2 and Figure 3 above indicate that the heart rate response in the shrimp did not match the heart rate increase that was initially expected. The explanation for the in-lab results may be that caffeine increase causes a decrease in heart rate in ghost shrimp, or there may be other factors that elicited this response and caffeine may have no effect at all. There are several possibilities for error that could have led to this outcome that must be taken into account. According to Defur and Mangum, a shrimp’s heart rate can be affected by its size, which means that our results may be skewed due to variability in the size of the shrimp used. Furthermore, outside stress factors were presented to the Palaemonetes paludosus as well.
The organisms were shipped to us from another habitat, and upon their arrival, the ghost shrimp were transferred into various containers for examination which may have put stress on the circulatory system or caused unexpected behavioral mannerisms in the arthropods. Thus, the actual numerical results could be skewed towards a higher observed heart rate. In addition, some of the specimen initially jumped out of their petri dishes simply as a response to being placed into an environmental gradient that differed from the norm. These external stress factors were taken into consideration when outlining the experiment. Aside from the knowledge gap surrounding this organism, the reason for choosing the ghost shrimp is because of its high stress tolerance.
Palaemonetes paludosus, like humans, have mechanisms for controlling circulatory patterns in a receptor-hormone secretion relationship. This relationship is dependent on an ionic level by calcium ions interacting with neuropeptides, such as proctolin in crustaceans, or epinephrine in humans. This same general schematic of ion/neuropeptide interaction in crustaceans is also observed in the Palaemonetes paludosus (Wilkens et al. 2005).
The adenosine receptor being blocked by caffeine is the instigating part of the mechanistic pathway that allows caffeine to affect humans (Smits et al. 1987). An experiment conducted by Celenza et al. (2007) placed crayfish into a solution containing varying concentrations of adenosine to test the pathway of adenosine uptake, but their experiment actually found no presence of adenosine receptors in crayfish; thus, along with supporting data in this experiment, it is highly probable that the same absence of an adenosine receptor is observed in ghost shrimp as well, indicating that the mechanism for how caffeine affects this particular organism must be different from that in humans. The relationship between adenosine receptors and caffeine makes it possible to hypothesize that heart rate will decrease or be unaffected as the concentration of caffeine increases, which differs from the initially proposed hypothesis and would be a subject for further studies.
 Further analyses of previous studies have demonstrated that increased uptake of caffeine allows more caffeine molecules to couple with free-floating ions in the cytosol. This coupling mechanism interacts with ryanodine receptors in the sarcoplasmic reticulum to create a release of intracellular calcium ions into the cytosol. Interestingly, the influx of calcium ions also inhibits sodium ions from binding to their designated ion channels, which results in a greater charge polarization on the membrane and less frequent action potentials, signaling the body to carry out a physical response (Celenza et al. 2007). The flow of sodium ions into the cell allows the membrane to depolarize. Even though the action potential still occurs, if sodium ions cannot bind to their channels, the rate at which the membrane depolarizes is diminished and it takes more time for the action to be completed.
Further analyses of previous studies have demonstrated that increased uptake of caffeine allows more caffeine molecules to couple with free-floating ions in the cytosol. This coupling mechanism interacts with ryanodine receptors in the sarcoplasmic reticulum to create a release of intracellular calcium ions into the cytosol. Interestingly, the influx of calcium ions also inhibits sodium ions from binding to their designated ion channels, which results in a greater charge polarization on the membrane and less frequent action potentials, signaling the body to carry out a physical response (Celenza et al. 2007). The flow of sodium ions into the cell allows the membrane to depolarize. Even though the action potential still occurs, if sodium ions cannot bind to their channels, the rate at which the membrane depolarizes is diminished and it takes more time for the action to be completed.
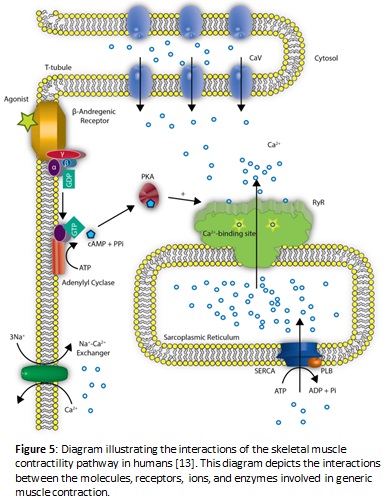
Another explanation for the decreased heart rate in ghost shrimp may derive itself from the overproduction of cAMP, a chemical messenger. The buildup of cAMP is hypothesized to be a result of caffeine binding to ryanodine receptors which releases calcium ions from the sarcoplasmic reticulum. The calcium ions can then directly interact with adenylyl cyclase to activate the enzyme and increase the conversion of ATP to cAMP. When this activation occurs, a buildup of some excess cAMP is present because it is being upregulated by calcium ions activating the enzyme behind the conversion. Decyclization of cAMP to AMP via the enzyme phosphodiesterase is also diminished as a result of caffeine exhibiting the ability to act as a competitive inhibitor against this enzyme. When excess cAMP is present, protein kinase A becomes upregulated and phosphorylates the sodium ion channels. When these channels become phosphorylated, this produces a relaxation effect in the smooth and cardiac muscles, and a decreased heart rate is observed. Relaxation will occur with greater magnitude in the presence of increasing concentrations of caffeine (Mustard 2014). A visual outline of the proposed biochemical pathway is represented in Figure 5.
The previous hypotheses are viable biochemical explanations for why the significantly decreased heart rate was prominent in shrimp held in solutions containing higher concentrations of caffeine for 10 minutes. However, the rationale for why the data shown in Figure 3 exhibited surprisingly increased heart rates in concentrations of 3 mg/ml and 7 mg/ml of caffeine/spring water solution after remaining in the solution for 10 minutes has not yet been discussed. The data received from the lowest two concentrations of caffeine in the lab does in fact match the previous research done on the crayfish (Mustard 2014). The causes for the increased heart rate at lower concentrations of caffeine are still uncertain and prior experiments have only grazed the surface of this biochemical phenomenon. The proposed hypothesis follows the thought process that caffeine somehow (directly or indirectly) inhibits adenylyl cyclase from being bound by specific activating G-proteins. Due to this inhibition, adenylyl cyclase can no longer convert ATP to cAMP as effectively and without the normal amount of cAMP present, it is unable to interact with the cAMP-dependent enzyme, protein kinase A, which causes the potassium ion channels to remain in an open state. When more of these ion channels are left open, action potentials can occur more frequently across the membrane; thus, a slight increase in activity is seen in that area. In the case of decreased heart rate in higher caffeine concentrations, the channels close due to the over-stimulation effect produced by the sudden excess of the secondary subcellular signaling messenger, cAMP; thus, relaxation in the muscles occurs, which may correlate with the decreased heart rate (Galligan 2014).
Future experimentation on the effects of caffeine on aquatic organisms or the relationship between those organisms and humans may include increasing the trials performed in each caffeine solution in the 10-minute exposure examination as well as experiments containing an adenosine solution to test for the presence or true absence of adenosine receptors in the Palaemonetes paludosus. If an adenosine receptor is actually present in the ghost shrimp, further testing on the adenosine receptors would be useful to compare the intake of adenosine and/or antagonistic molecules to how they are received in vertebrates. Thus, additional understanding will be gained on the similarities and differences between invertebrates’ hormonal and neurological biochemical processes and that of humans.
References:
1. Ogawa, N. and Ueki, H. 2007. Clinical importance of caffeine dependence and abuse. Psychiatry Clin Neurosci. 61(3): p. 263-268.
2. Fredholm, B.B., et al. 1999. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 51(1): p. 83-133.
3. Marangos, P.J., Boulenger, J.P., and Patel, J. 1984. Effects of Chronic Caffeine on Brain Adenosine Receptors – Regional and Ontogenetic Studies. Life Sciences. 34(9): p. 899-907.
4. Smits, P., et al. 1987. Evidence for an Antagonism between Caffeine and Adenosine in the Human Cardiovascular System. J Cardiovasc Pharmacol. 10(2): p. 136-143.
5. Mustard, J.A. 2014. The Buzz on Caffeine in Invertebrates: Effects on Behavior and Molecular Mechanisms. Cell Mol Life Sci. 71(8): p. 1375-1382.
6. Phillips, P. and Chalmers, A. 2009. Wastewater Effluent, Combined Sewer Overflows, and Other Sources of Organic Compounds to Lake Champlain. Journal of the American Water Resources Association. 45(1): p. 45-57.
7. Moore, M.T., et al. 2008. Assessing Caffeine as an Emerging Environmental Concern using Conventional Approaches. Arch Environ Contam Toxicol. 54(1): p. 31-5.
8. Garcia, R.N., et al. 2014. Individual and Mixture Effects of Caffeine and Sulfamethoxazole on the Daggerblade Grass Shrimp Palaemonetes pugio following Maternal Exposure. Environ Toxicol Chem. 33(9): p. 2120-2125.
9. Defur, P.L. and Mangum, C.P. 1979. Effects of Environmental Variables on the Heart-Rates of Invertebrates. Comparative Biochemistry and Physiology a-Physiology. 62(2): p. 283-294.
10. Wilkens, J.L., et al. 2005. Sites and Modes of Action of Proctolin and the FLP F2 on Lobster Cardiac Muscle. J Exp Biol. 208(Pt 4): p. 737-747.
11. Celenza, K.M., Shugert, E., and Velez, S.J. 2007. Depressing Effect of Caffeine at Crayfish Neuromuscular Synapses II. Initial Search for Possible Sites of Action. Cell Mol Neurobiol. 27(3): p. 381-393.
12. Elmenhorst, D., et al. 2007. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 27(9): p. 2410-2415.
13. Zhiguang Yuchi, Lynn Kimlicka and Filip Van Petegem (2013). Structural Insights Into Disease Mutations of the Ryanodine Receptor, Genetic Disorders, Prof. Maria Puiu (Ed.), ISBN: 978-953-51-0886-3, InTech, DOI: 10.5772/53641. Available from: http://www.intechopen.com/books/genetic-disorders/structural-insights-into-disease-mutations-of-the-ryanodine-receptor
Acknowledgments: Ashley Smith and Tori Fabacher assisted in the research for this experiment as well as participating in the procedure designed by the team. Thank you to Elise White, who helped with the grammatical and syntactical revisions for this research paper. A special thank you to Amy Styer Greene, the GLA for this Biology lab section, who devoted much of her time assisting in the experimental setup and revision processes.
Citation style: Parenthetical citations are in the numbered style and the works cited page is consistent with the suggested scientific style found in the BIOL 1107L/BIOL 2107L Principles of Biology I Laboratory Manual: Second Edition: (pg. 5354).