A Synthesis of Current Research on The Evolutionary Relationship Between Sickle Cell
Disease and Malaria
by Ellie Halpert, Genetics
This paper provides a comprehensive review of the current research concerning the evolutionary relationship between sickle cell disease and malaria, a classic example of genetic adaptation to environmental pressures. Sickle cell disease, resulting from mutations in the hemoglobin gene (HBB), has been found to offer a survival advantage against malaria in individuals who carry the sickle cell trait. This review synthesizes key findings from several landmark studies, tracing the global distribution of sickle cell alleles and their evolutionary origins in response to malaria.
The paper explores the protective mechanisms conferred by different hemoglobin variants, including HbS, HbC, and HbE, against malaria, with a focus on how these variants have evolved independently in different populations. Additionally, there is an examination of the immune and mechanical changes in red blood cells associated with the sickle cell trait, which confer protection against malaria-causing Plasmodium parasites.
Recent research highlights the interaction between malaria and sickle cell disease, including emerging insights into host immune responses and the varying degrees of protection offered by hemoglobin variants. The review also discusses the clinical and evolutionary implications of this interaction and points to future research directions in this field. Understanding these genetic adaptations is crucial for public health strategies aimed at controlling malaria in endemic regions.
sickle cell disease, anemia, malaria, genetics, evolutionary biology
Introduction
The intriguing link between malaria and sickle cell disease has been a subject of intense research for decades. Malaria, caused by a variety of Plasmodium parasites, has been a major selective force shaping the human genome. Malaria is the evolutionary driving force behind sickle cell disease, thalassemia, glucose-6-phosphatase deficiency, and other red blood cell defects that comprise the most common Mendelian diseases of humankind (Kwiatkowski et al., 2005). One of the most well-studied examples of this interaction is the relationship between sickle cell disease and malaria resistance. This synthesis will explore key findings and insights from several leading papers that have contributed to our understanding of how the sickle cell trait has evolved as a genetic adaptation in conjunction with malaria.
A foundational paper published by Anthony C. Allison in 1960 laid the groundwork for understanding the genetic basis of malaria resistance. Allison’s work highlighted the prevalence of the sickle cell trait (HbAS) in regions where malaria is endemic. He proposed that heterozygous individuals, who carry one normal hemoglobin allele (HbA) and one sickle hemoglobin allele (HbS), had a survival advantage against malaria, when compared to individuals with either HbA or HbS homozygosity (two copies of the same allele). This idea is now known as the Malaria Hypothesis. Another foundational study published my Kwiatkowski et al. (2005) observed that homozygotes for the allele (HbSS) suffer from sickle cell anemia
(SCA), but heterozygotes (HbAS) do not suffer from SCA and have a 10-fold reduced risk of severe malaria.
Allison’s paper provided the first compelling evidence for the selective advantage of HbAS in malarial regions, sparking further research into the mechanisms driving this relationship. These landmark studies collectively shed light on the role of the sickle cell trait in conferring resistance to malaria in human populations, and the evolution of this phenomenon over time.
Current Knowledge
Geographic Distribution and Epidemiology:
A strong geographic correlation has been established between the sickle cell gene and malaria prevalence, supporting the idea that the sickle cell trait has evolved as a protective adaptation in response to malaria’s selective pressure. A meta-analysis of studies on how different types of hemoglobin protect against malaria found that the sickle cell trait (HbAS) consistently offered protection. Hemoglobin is a protein in red blood cells that carries oxygen. Different versions, called alleles, of the hemoglobin gene exist, such as HbS, HbC, and HbE, which protect against malaria in different ways. Genetic variations, like HbAC and HbCC, showed some defense against severe malaria, though to a lesser extent (Taylor et al., 2012). This analysis found that only HbAS was consistently associated with protection from uncomplicated malaria. This trend led to the conclusion the HbAS, HbCC, and HbAc genotypes, as well as the presence of at least one a-thalassemia allele, provided significant protection against severe malaria, but they varied greatly in the degree of protection against uncomplicated or asymptomatic malaria (Taylor et al., 2012). Their findings emphasize the role of the sickle cell trait as a long-standing adaptation in malaria-endemic areas. Although diverse in variants and degree, Taylor et al. found that the protective effect provided by the a-thalassemia allele is consistent across different regions and populations where malaria is endemic.
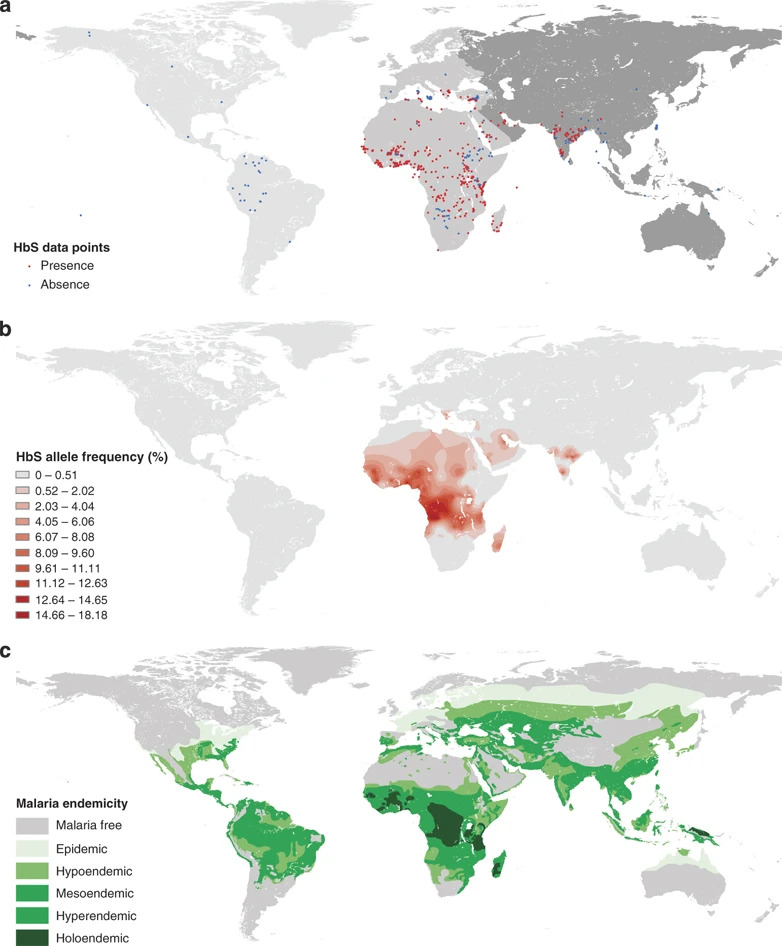
Understanding the global distribution of the sickle cell gene and its impact on malaria epidemiology is crucial for public health interventions. Piel et al. (2010) mapped the global distribution of the sickle cell gene, confirming its highest prevalence in malaria-endemic regions. The analysis provided by Piel at al. indicates a strong geographic correlation between malaria’s endemicity and the HbS allele’s presence. This correlation supports the “malaria hypothesis,” suggesting that HbS has evolved as a protective genetic adaptation in response to malaria that is more common in malaria-endemic regions due to the selective advantage it provides against the disease (Piel et al., 2010). This work provided a geographical context for the malaria hypothesis and emphasized the importance of considering regional genetic factors in malaria control strategies.
Figure 1. Global Distribution of the HbS allele. (a) Distribution of the presence of the HbS allele. (b) HbS allele frequency distribution. (c) Historical map of severity of malaria endemicity. Piel et al. (2010)
Genetic Variants
After the initial research focusing on the HbS allele, several researchers set out to understand the variety of hemoglobin alleles protected against malaria parasites. The idea that other hemoglobin variants, such as HbC and HbE, can also confer protection against clinical malaria, was first introduced in 2005 and suggests that multiple genetic adaptations likely arose independently in different populations where malaria is endemic (Kwiatkowski et al., 2005). This discovery helps us understand how different versions of hemoglobin evolved to protect against malaria. The strong evolutionary pressure from malaria has caused the HbS gene to become common in regions with high malaria rates, even though having two copies of the gene can be fatal (Kwiatkowski et al., 2005). Different populations have independently evolved different genetic responses to malaria, as seen in the variety of alleles that provide degrees of protection. Researchers have identified three genetic changes in the HBB gene, which makes hemoglobin. The HBB gene contains small changes called single nucleotide polymorphisms (SNPs). These genetic variations create different forms of hemoglobin: HbS, HbC, and HbE. Each hemoglobin form provides some protection against malaria and is associated with general regions: HbS is common in Africa, HbC is found in parts of West Africa, and HbE is common in Southeast Asia. For example, the Dogon people in Mali have more HbC and less HbS than other groups in West Africa. The HbS gene itself has evolved differently in different populations, showing its importance in human adaptation to malaria. (Kwiatkowski et al., 2005).
The use of hemoglobin electrophoresis to divide study participants into groups based on phenotype – HbAA, HbAS, and HbSS – effectively allowed researchers to evaluate the degree of resistance to malaria by each hemoglobin genotype (Makani et al., 2010). Prior to their study, Allison (1960) had proven that heterozygotes (HbAS) are protected against malaria. Makani et al.’s study revealed a lower prevalence of malaria parasitemia in patients with the homozygous HbSS genotype (Makani et al., 2010). These results led to further questions about the dose-dependency of the HbS allele, as patients with SCA have higher levels of the protective allele against malaria. Previous research on the allele HbC concluded that there is considerably greater protection against malaria for homozygous (HbCC) individuals when compared to
heterozygous (HbAC) individuals (Kwiatkowski et al., 2005). Having two copies of the HbC gene (HbCC) results in a less severe form of sickle cell disease compared to having two HbS genes (HbSS). However, both the HbCC and HbAC (one HbC gene and one normal gene) forms protect against severe malaria.
Mechanisms of Malaria Protection by SCA
Research shows that people with the sickle cell trait have changes in their red blood cells, as well as an improved immune response that helps protect against malaria (Williams et al., 2005). In one study in Kenya, children with the sickle cell trait were 40% less likely to get mild malaria, with protection being strongest in early childhood. The researchers propose several mechanisms that allow for the protective effects of the HbAS genotype. The accelerated acquisition of antibodies to altered host antigens is an observed interaction with the HbAS red blood cells. Alternatively, by controlling parasite densities during malaria infections, the HbS allele may paradoxically increase the chronicity of individual infections, which reduces their severity (Williams et al., 2005). This demonstration of an altered immune response to Plasmodium infection, leading to a more effective defense against the parasite, complements the mechanical changes in red blood cells associated with sickle hemoglobin. The study highlights the multifaceted nature of protection conferred by the sickle cell trait, proposing that this protection is not solely due to the physical changes in red blood cells caused by sickle hemoglobin, but is also related to the immune response (Williams et al., 2005).
In 2011, Ferreira et al. expanded upon this research by revealing that HbAS individuals exhibited an enhanced tolerance to Plasmodium infection despite carrying the parasite at densities similar to that of HbAA individuals. The study proposed that this tolerance was due to the preferential clearance of infected red blood cells in HbAS individuals, limiting the severity of the disease. The study published by Ferreira et al. addresses the longstanding observation that individuals with sickle cell trait (one copy of the sickle hemoglobin gene) have a reduced susceptibility to severe malaria compared to individuals with normal hemoglobin. Further, the study expands into the mechanism behind this reduced susceptibility and proposes that sickle hemoglobin confers tolerance to Plasmodium infection rather than preventing it altogether. Researchers used mice with HbS to demonstrate a distinct immune response to Plasmodium infection. The results of this experiment reveal that, relative to HbA mice, HbS mice experience less severe malaria-related symptoms, lower parasitemia (parasite levels in the blood), and a decreased inflammatory response compared to mice with normal hemoglobin (Ferreira et al., 2011). These differences suggest that sickle hemoglobin reduces the availability of heme, which is essential for the growth of Plasmodium parasites, thus limiting their replication.
Next Steps
Clinical Implications and Future Directions
The clinical implications of the malaria-sickle cell interaction have been a topic of ongoing research. Kwiatkowski (2005) provided a comprehensive overview of how malaria has influenced the human genome and highlighted the potential for human genetics to inform malaria research and control strategies. Makani et al. (2010) conducted a study specifically focused on malaria in patients with sickle cell anemia (SCA) in Dar-es-Salaam, Tanzania. The focus of this study was to examine malaria’s burden, risk factors, and outcomes in individuals with SCA. Researchers found that during hospitalization, patients with the HbAS genotype had a higher rate of malaria events and parasitemia associated with higher mortality, unveiling the need for tailored interventions to manage malaria in this vulnerable population. The host response to malaria in children with sickle cell anemia and severe anemia has more recently been explored in a study performed at Mulago Hospital in Uganda, with children between 18 months and 12 years old (Henrici et al., 2021). Several key findings resulted from this study. Children with severe anemia and P. falciparum parasitemia exhibited differences in parasite sequestration, inflammatory responses, and endothelial cell dysfunction based on whether they had HbSS, HbSA, or HbAA. These results suggested that impaired cytoadherence (binding of infected red blood cells to endothelial cells) in children with SCA might explain decreased parasite sequestration. The study hints at potential long-term consequences from severe malaria if endothelial and dysregulation persist after the malaria episode, in children with SCA (Henrici et al., 2021). Their research revealed altered host responses in these individuals, suggesting that the interaction between malaria and SCA is more complex than previously believed and requires further research.
Implications for Evolutionary Studies
The link between sickle cell disease (SCD) and protection against malaria is a great example of how diseases can shape human genetics over time. While the protective effect of the primary sickle cell trait against malaria is well-established (Makani et al., 2010), questions remain regarding the selective pressures driving its prevalence and whether other genetic factors, in addition to HbAS, may contribute to malaria resistance. Investigating these factors in diverse populations could provide a more nuanced understanding of human adaptation to malaria. Sickle cell disease is one example of how hemoglobinopathies may offer resistance to malaria infection. Other hemoglobin variants, such as HbC and HbE, also provide some protection (Kwiatkowski et al., 2005). Further research is needed to explore the genetic and evolutionary aspects of these variants, as well as their interactions with malaria in the human body. The prevalence of HbAS and other hemoglobin variants, fluctuates significantly across malaria-endemic regions (Kwiatkowski et al., 2005). Investigating the regional differences in the distribution of sickle cell alleles and their relationship with distinct malaria strains is crucial to developing region-specific malaria control strategies.
Studying the long-term evolutionary dynamics of the sickle cell gene can provide insights into the coevolution of humans and Plasmodium parasites. Analyzing ancient DNA and conducting population genetics studies could help reconstruct the historical trajectories of these genetic adaptations. At the same time, as genetic research advances, there is a growing need to address the ethical and social implications of genetic interventions, such as genome editing, to modify the prevalence of SCD in malaria-endemic regions. The potential for unintended consequences and ethical dilemmas stresses the importance of holistic research approaches—considering both scientific and societal aspects.
The evolution of the sickle cell trait in response to malaria is a fascinating case study in human genetics and disease resistance. While substantial progress has been made in evaluating this genetic adaptation’s mechanisms and epidemiological impact there are still significant gaps in our knowledge. Addressing these gaps via interdisciplinary research will enhance our understanding of human evolution and contribute to more effective malaria control strategies. Furthermore, researchers must remain mindful of the ethical and societal implications of their work as they navigate the intersection of genetics, disease, and public health.
Conclusion
Together, the research examined in this study shed light on the multifaceted mechanisms underlying the protective effect of HbAS against malaria from reduced parasite adhesion to enhanced host tolerance. The evolution of the sickle cell trait in response to malaria is a compelling example of how infectious diseases have shaped human genetic diversity. The research discussed in this synthesis collectively provides a comprehensive understanding of the genetic, molecular, epidemiological, and clinical aspects of the malaria-sickle cell interaction. This knowledge not only contributes to our appreciation of human evolution but also informs public health efforts to combat malaria in regions where it remains a significant threat. Recent studies, such as the study published by Henrici et al. (2021), emphasize the complex relationship between sickle cell disease and malaria. Further research in this field, especially utilizing genetic technology advances, promises to yield valuable insights into both the biology of malaria and the
genetic adaptations that have arisen in response to it.
References
Allison, A. C. (1960). Genetic control of resistance to human malaria. Current Anthropology,
1(4), 283-288.
Ferreira, A., Marguti, I., Bechmann, I., Jeney, V., Chora, A., Palha, N. R., … & Soares, M. P.
(2011). Sickle hemoglobin confers tolerance to Plasmodium infection. Cell, 145(3),
398-409.
G., Marsh, K., & Williams, T. N. (2010, January 14). Malaria in patients with sickle cell anemia:
Burden, risk factors, and outcome at the outpatient clinic and during hospitalization.
American Society of Hematology. https://doi.org/10.1182/blood-2009-07-233528
Henrici, R. C., Sautter, C. L., Bond, C., Opoka, R. O., Namazzi, R., Datta, D., Ware, R. E.,
Conroy, A. L., & John, C. C. (2021, November 18). Decreased parasite burden and
altered host response in children with sickle cell anemia and severe anemia with malaria.
American Society of Hematology. https://doi.org/10.1182/bloodadvances.2021004704
Kwiatkowski, D. P. (2005). How malaria has affected the human genome and what human genetics can teach us about malaria. American journal of human genetics, 77(2), 171–192. https://doi.org/10.1086/432519
Makani, J., Komba, A. N., Cox, S. E., Oruo, J., Mwamtemi, K., Kitundu, J., Magesa, P., Rwezaula, S., Meda, E., Mgaya, J., Pallangyo, K., Okiro, E., Muturi, D., Newton, C. R., Fegan, G., Marsh, K., & Williams, T. N. (2010). Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood, 115(2), 215–220. https://doi.org/10.1182/blood-2009-07-233528
Piel, F. B., Patil, A. P., Howes, R. E., Nyangori, O. A., Gething, P. W., Dewi, M., … & Hay, S. I.
(2010). Global distribution of the sickle cell gene and geographical confirmation of the
malaria hypothesis. Nature Communications, 1(1), 1-7.
Taylor, S. M., Parobek, C. M., Fairhurst, R. M. (2012). Haemoglobinopathies and the clinical
epidemiology of malaria: a systematic review and meta-analysis. The Lancet Infectious
Diseases, 12(6), 457-468.
Williams, T. N., Mwangi, T. W., Roberts, D. J., Alexander, N. D., Weatherall, D. J., Wambua, S.,
… & Marsh, K. (2005). An immune basis for malaria protection by the sickle cell trait.
PLOS Medicine, 2(5), e128.
Citation Style: APA