Efficacy of neutrophil elastase inhibitors in chronic lung disease patients:
A systematic review
by Yeongseo Son, Biochemistry and Molecular Biology
Abstract: Though treatments for chronic lung diseases exist, a definitive cure for lung damage remains elusive. Elevated neutrophil levels and neutrophil elastase (NE) activity exacerbate lung inflammation. Over two decades, several NE inhibitors emerged to address this issue. This review extensively evaluates the existing literature on NE inhibitors’ efficacy in three chronic lung diseases: cystic fibrosis, chronic obstructive pulmonary disease, and bronchiectasis. From September to October 2023, clinical trials published on PubMed between 2000 and 2023 were searched, with the primary outcomes collected being the evaluation of lung function improvement and the secondary outcome focused on NE inhibitors’ safety. After evaluation of research containing fully completed clinical trials, 12 studies were included in the literature review, and five different NE inhibitors were identified. Only two (AZD9668 and Alpha1-Antitrypsin) underwent multiple trials. Notably, they had statistically significant results that indicated improvements in patients’ inflammatory biomarkers. Overall, trials with NE inhibitors in chronic lung conditions showed limited clinical improvements in lung function. Some studies have suggested that short trial durations and low dosages might contribute to these outcomes. Future studies should investigate NE inhibitors’ ability to effectively inhibit NE in humans before exploring their clinical benefits for patients.
neutrophil elastase, cystic fibrosis, chronic obstructive pulmonary disease, bronchiectasis, clinical trials
Introduction
Respiratory conditions encompass various ailments affecting the airways and lungs. Within this spectrum, chronic lung diseases are persistent illnesses that significantly impact lung function and health over extended periods. Notably, cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and bronchiectasis present a global challenge and contribute yearly to approximately four million premature deaths.1-4 Despite their varying causes and symptoms, these three conditions share an underlying factor: persistent lung inflammation leading to irreversible damage. While various treatments exist, such as modulator therapies for CF patients and antibiotics for bronchiectasis-related infections, a cure for lung damage remains elusive.
Characterized by thick mucus obstructing the airway and digestive systems, CF results from an inherited mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) protein.5 In contrast, COPD and bronchiectasis are primarily acquired conditions. COPD arises from prolonged exposure to irritants, contributing to airflow obstruction and increased mucus production.6 Bronchiectasis stems from recurrent infections that lead to a permanent widening of the lungs.7 In all three conditions, elevated neutrophil levels play a pivotal role in exacerbating lung inflammation.
Role of Neutrophils and Neutrophil Elastase
Neutrophils, a type of white blood cells, constitute the body’s initial line of defense against bacterial and fungal pathogens through phagocytosis.8,9 However, in chronic conditions, these immune cells continually release their toxic granule contents, such as the serine protease neutrophil elastase (NE).
Stored within the granules of neutrophils, NE induces immune responses when released by neutrophils. It can facilitate leukocyte transmigration to the site of infection and clear gram-negative bacteria by cleaving their outer membrane proteins.10 However, excess NE activity can disrupt lung architecture and stimulate persistent inflammation.11,12 Increased NE activity is strongly involved in the cascade of pathogenic events that occur in CF, COPD, and bronchiectasis patients. In fact, amplified NE activity in bronchial lavage fluid closely correlates with progressive structural lung damage even in CF children,10 underscoring NE’s significant impact on the progression of chronic lung diseases.
In Vitro and In Vivo Studies on NE Inhibitors
To protect the body from excessive inflammation, innate NE inhibitors, like elafin and secretory leukocyte protease inhibitors, are produced. They bind to NE and prevent the protease from interacting with its target substrates.8 To replicate these natural defenses, various NE inhibitors have been identified and tested in different respiratory diseases. These inhibitors include Alpha1-Antitrypsin (AAT), sivelestat, ecotin, AZD9668, EPI-hNE4, monocyte NE inhibitor, KRP-109, BAY 85-8501, POL6014, Alpha1-proteinase inhibitor (Alpha1-PI), and DX-890.13,14
While AAT, AZD9668, BAY 85-8501, POL6014, and Alpha1-PI have advanced to clinical trials, the majority have been primarily studied in in vitro and in vivo animal models. For instance, both ecotin and DX-890 have significantly reduced NE activity in neutrophils isolated from CF and healthy subjects.14,15 Additionally, in animal models of chronic lung diseases, studies have consistently discovered a decrease in both NE activity and alveolar inflammation following NE inhibitor treatments.13 For example, mice with respiratory damage exhibited diminished clinical signs of lung injury after sivelestat administration, a small molecule inhibitor of NE.16 These collective findings highlight the potential of NE inhibitors in managing chronic lung diseases by lowering lung inflammation.
Clinical Trials on NE Inhibitors
Clinical trials investigating NE inhibitors have primarily focused on patients with chronic lung conditions such as CF, COPD, and bronchiectasis. Earlier clinical studies focused on the tolerability of the treatment in different dosages while later studies measured inflammatory biomarkers and lung function.17,18,20 While systematic literature reviews exist for the effectiveness of NE inhibitors in animal models of lung conditions,13 no reviews have provided an overview of the efficacy of NE inhibitors on patients with chronic lung conditions.
Objectives
Given the limited reviews on NE inhibitors and their impact on chronic lung diseases, the main objective of this systematic literature review is to provide a comprehensive summary of clinical trials about NE inhibitors, with a focus on evaluating their efficacy in improving lung function.
Methods
Search Strategy and Screening
The electronic database PubMed was searched. The initial literature search in September 2023 employed the terms “neutrophil elastase inhibitors,” “reducing neutrophil elastase levels,” “neutrophil inhibitors,” and “cystic fibrosis.” The applied filters included clinical studies, clinical trials, and controlled clinical trials. Only studies published in English were considered. Due to limited results, the search was broadened to encompass two more chronic lung conditions: chronic obstructive pulmonary disease and bronchiectasis. While the original literature search covered studies from 2013 to 2023, the updated criteria broadened the scope to include pertinent papers dating back to 2000. This extension allowed for an exhaustive examination of studies on neutrophil elastase inhibitors published from 2000 to September 2023. All identified studies were systematically organized in Mendeley, and duplicates were removed.
Eligibility Criteria
Population
In this review, subjects of all ages, sexes, and races afflicted with cystic fibrosis, chronic obstructive pulmonary condition, or bronchiectasis were included (Supplementary Table 1). There were no restrictions based on geographical location. Subjects with varying disease severities, ranging from mild to severe presentations, were included. Having more than one of these conditions did not serve as an exclusion criterion.
Intervention
Studies that examined the effectiveness of neutrophil elastase inhibitors in improving lung function were included. Studies unrelated to chronic lung conditions were excluded. Additionally, trials involving unrelated interventions such as surgeries and lung transplants, were not considered.
Study Design
This review incorporated literature with randomized clinical trials in any phase. Excluded were reviews, research protocols, conference abstracts, ongoing studies, case studies, opinions, and commentaries. These sources, while informative, did not provide the data required for reporting on the impact of neutrophil elastase inhibitors on lung function. The clinical trials considered were inclusive in their choice of controls, including placebos, no interventions, healthy subjects, or no controls.
Data Collection
Participant characteristics, including sample size, age, sex, race, and type of chronic lung condition, were included. Furthermore, descriptive study characteristics were documented, such as the study design, the duration of therapy, the treatment dosages, and the variables measured. The primary outcome of interest centered on changes in lung function, assessed through both standardized and non-standardized evaluations. Standardized assessments involved well-established tests like FEV1 (Forced Expiratory Volume in 1 second) and measurements of lung volume. Meanwhile, non-standardized assessments included quality of life and symptom-based questionnaires. The secondary outcome measured was the safety of the neutrophil elastase inhibitors, which was assessed by quantifying major adverse events and side effects. Lastly, missing data, such as incomplete outcome measures and participant dropouts, were reported in the literature reviewed.
Results
Search and Selection of Studies
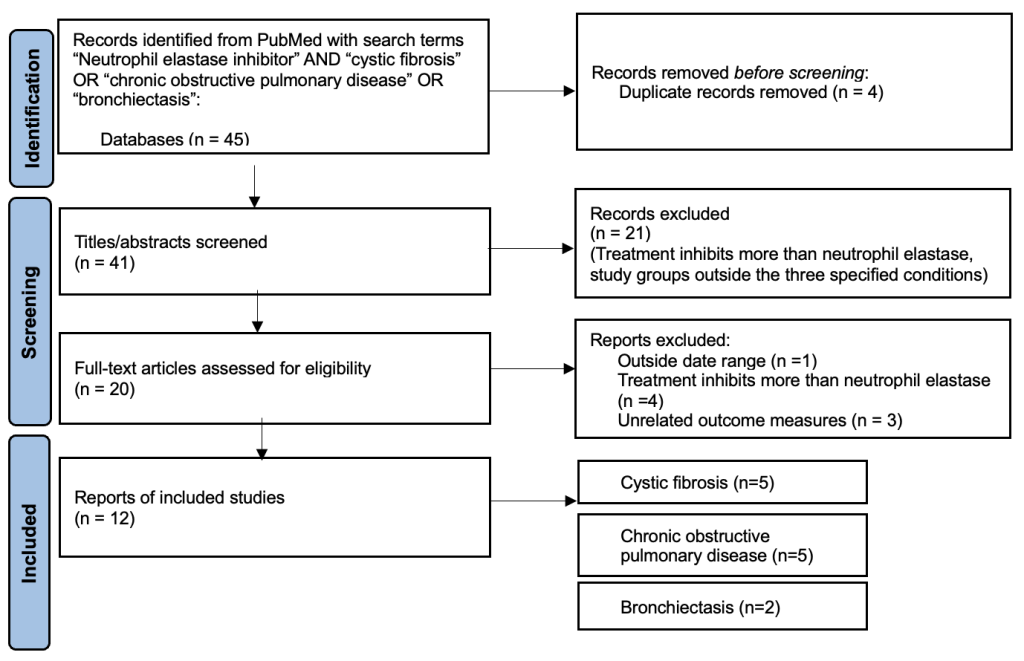
Out of the 45 studies identified through the literature search, 20 met the inclusion criteria based on title and abstract. After evaluation of the full texts, 12 papers remained. Studies were mostly excluded due to the following reasons: treatment inhibited proteins that were not neutrophil elastase, or the study measured outcomes unrelated to lung function (Supplementary Figure 1).
Publication years spanned 2006 to 2020, with a concentration around 2012 (Supplementary Table 2). Studies were conducted across Europe, Asia, and North America. While some studies were conducted in a single area, other studies encompassed multiple sites. The most prominent study sites were Germany (n=8) and the United Kingdom (n=7).
Study Characteristics
Five NE inhibitors that underwent clinical trials were identified: AZD9668, BAY 85-8501, POL6014, Alpha1-Antitrypsin (AAT), and Alpha1-proteinase inhibitor (Alpha1-PI). AZD9668 had the most studies conducted (n=6), whereas BAY 85-8501, POL6014, and Alpha1-PI each underwent a single trial (Table 1). Although all trials employed randomization, they incorporated different designs: three studies included a healthy control group, and ten used placebo-controlled groups. The outcomes measured also varied, with eleven studies using common lung function parameters, such as FEV1.1
Seven studies used inflammatory biomarkers, or molecules that indicate the presence and severity of inflammation, to study the effects of the treatment in the patients. Some commonly measured inflammatory biomarkers in these trials included C-reactive protein and interleukins (IL-6 and IL-8).17,25 These biomarkers helped researchers gauge the effectiveness of NE inhibitors in reducing inflammation associated with respiratory diseases.
Table 1. Methodological characteristics of included studies (n=12)
Table 1A. AZD9668 Studies (n=6)
| Studies | Design | Total size | Condition | Dosage | Duration (weeks) | Outcome measurements |
| Stockley et al.17 | Phase II, randomized, double-blind, placebo-controlled, parallel-group trial | 38 | Bronchiectasis | 60 mg bid | 4 | Waking and post-waking sputum; neutrophil counts; lung function tests; 24-h sputum weight; BronkoTest diary card data; SGRQ-C; sputum NE activity; inflammatory biomarker levels; desmosine levels; adverse events, safety hematology and biochemistry; AZD9668 levels in plasma and sputum |
| Elborn et al.25 | Phase II, randomized, double-blind, placebo-controlled trial | 56 | CF | 60 mg bid | 4 | Sputum neutrophil count; lung function; 24-h sputum weight; BronkoTest® diary card data and health-related quality-of-life; sputum neutrophil elastase activity; inflammatory biomarkers in sputum and blood, urine and plasma desmosine; AZD9668 levels; adverse events, sputum bacteriology |
| Vogelmeier et al.24 | Phase IIb, randomized, double-blind, placebo-controlled trial | 838 | COPD | 5, 20, and 60 mg bid | 12 | Pre-bronchodilator FEV1; forced vital capacity and inspiratory capacity; peak expiratory flow; Breathlessness, Cough and Sputum Scale (BCSS); adverse event; exercise capacity; quality of life (QoL); exacerbation assessments; safety and pharmacokinetics; inflammatory and tissue degradation biomarkers |
| Kuna et al.23 | Phase IIb, randomized, double-blind, placebo-controlled trial | 615 | COPD | 60 mg bid | 12 | FEV1; post-bronchodilator FEV1; pre- and post-bronchodilator forced vital capacity; FEV6; Cough and Sputum Scores; adverse event; St George’s respiratory questionnaire for COPD (SGRQ-C) scores; exacerbations; safety assessments |
| Gunawardena et al.19 | Randomized, double-blind, placebo-controlled trial | 125 | COPD | 60 mg bid | 2 | Plasma concentration profiles of AZD9668; FEV1; adverse event |
| Nordenmark et al.26 | Phase IIb, randomized, double-blind, placebo-controlled trial | 52 | COPD | 60 mg bid | 12 | Airway wall thickness at an extrapolated interior perimeter of 10 mm (AWT-Pi10); fifth-generation wall area %; air trapping index; pre- and post-bronchodilator FEV1; morning and evening peak expiratory flow and FEV1; adverse event; body plethysmography; Breathlessness, Cough, and Sputum Scale (BCSS); safety variables |
Table 1B. AAT studies (n=3)
| Studies | Design | Total size | Condition | Dosage | Duration (weeks) | Outcome measurements |
| Martin et al.21 | Phase II, randomized, double-blinded, placebo-controlled trial | 39 | CF | 500, 250, and 125 mg daily | 6 | Sputum NE activity; sputum myeloperoxidase (MPO), interleukin-8, tumor necrosis factor receptors, sputum and plasma NE/AAT complexes; safety parameters; adverse event |
| Griese et al.22 | Randomized, open-label, parallel group trial | 52 | CF | 25 mg daily | 4 | NE activity; pro-inflammatory cytokines, neutrophils, immunoglobulin G fragments; Pseudomonas aeruginosa; adverse event |
| Stolk et al.28 | Randomized, double-blinded, parallel group trial, placebo-controlled trial | 168 | COPD | 80 mg bid | 50 | Time from randomization to the first event-based exacerbation; safety; adverse event |
Table 1C. BAY 85-8501 studies (n=1)
| Studies | Design | Total size | Condition | Dosage | Duration (weeks) | Outcome measurements |
| Watz et al.27 | Phase IIa, randomized, double-blind, placebo-controlled, parallel-group trial | 94 | Bronchiectasis | 1 mg daily | 4 | Treatment-emergent adverse events; FEV1; health-related quality of life; SGRQ total score |
Table 1D. POL6014 studies (n=1)
| Studies | Design | Total size | Condition | Dosage | Duration | Outcome measurements |
| Barth et al.29 | Randomized, double-blind, placebo-controlled, parallel-group trial | 24 | CF | 80, 160, and 320 mg bid | 1 day | FEV1; safety, tolerability and pharmacokinetics (PK) of single ascending doses of inhaled POL6014 with a Pari eFlow® nebulizer; NE activity; adverse event |
Table 1E. Alpha1-PI studies (n=1)
| Studies | Design | Total size | Condition | Dosage | Duration | Outcome measurements |
| Häussermann et al.20 | Randomized, uncontrolled, cross-over, single-dose study | 21 | CF | 77 mg | 2 days | Extra thoracic deposition; exhaled drug fraction; nebulizer residue; adverse event |
Patient Characteristics
Most NE inhibitor clinical trials focused on CF (n=5) and COPD (n=5). CF patients tended to be younger with a mean age of 28.6 years, whereas COPD and bronchiectasis patients on average ranged near 60 years old (Supplementary Table 3). In terms of gender distribution, every study that provided this information had higher representation of males than females. Notably, three studies did not include data on the patients’ race. Among the included studies, six exclusively involved Caucasian participants, whereas three studies included participants from other races.
Risk of Bias
Although all studies were randomized, two20,22 lacked double blinding, which could potentially introduce bias. Double blinding helps to maintain objectivity and ensures that any observed effects are more likely to be due to the treatment itself. Furthermore, two studies20,29 had small sample sizes (fewer than 30 subjects), which may leave room for bias due to limited representation and confounding variables. Lastly, certain studies received direct funding from the drug company, such as AZD9668, potentially introducing further bias.
Outcome of the Studies
NE inhibitors’ efficacy was measured in various ways. The most common assessments were neutrophil count, NE activity, inflammatory biomarkers, FEV1, and pre/post-treatment lung function.
Table 2. Primary outcomes that are statistically significant P<.05
| Treatment | Studies | Neutrophil count | NE activity | inflammatory biomarkers | FEV1 | Lung Function |
| AZD9668 | Elborn et al.25 | X | X | O | X | X |
| Vogelmeier et al.24 | – | – | X | X | X | |
| Kuna et al.23 | – | – | X | X | X | |
| Gunawardena et al.19 | – | X | – | – | – | |
| Stockley et al.17 | X | X | O | O | X | |
| Nordenmark et al.26 | – | – | – | X | X | |
| BAY 85-8501 | Watz et al.27 | X | O | X | X | X |
| POL6014 | Barth et al.29 | – | X | – | X | – |
| AAT | Martin et al.21 | – | X | O | X | X |
| Griese et al.22 | O | O | O | X | X | |
| Stolk et al.28 | – | – | – | X | X | |
| Alpha1-PI | Häussermann et al.20 | – | – | – | X | X |
Overall, five studies saw statistically significant improvements with NE inhibitor treatment (Table 2). The most prevalent statistically significant improvement observed in patients was inflammatory biomarker reduction. Only one study, which involved AZD9668 treatments with bronchiectasis patients, observed significant improvements in FEV1.17 On the other hand, most of the studies (n=7) reported non-statistically significant results that indicated lung function improvement (Supplementary Table 4). The most frequently recorded positive outcomes were NE activity reduction and reduction of inflammatory biomarkers.
Lastly, safety of NE inhibitors was evaluated by assessing dropout rates and adverse events post-treatment. No fatalities occurred after the treatments, but all studies reported adverse effects (Table 3). Adverse events were systematically monitored and constituted any unforeseen or undesirable incident. These events included side effects linked to the treatment, the worsening of existing conditions, and the emergence of new symptoms. The dropout rates and adverse events exhibited variability among different treatments as well as between trials assessing the same treatment. For instance, adverse events associated with AZD9669 ranged from 11% to 68%. Most studies reported adverse events after treatments included coughing, headaches, and nasopharyngitis.
Table 3. Incidence of dropouts and adverse events
| Treatment | Studies | Reported dropout after treatment | Adverse events reported after treatment | Adverse effects reported |
| AZD9668 | Elborn et al.25 | 0 (0%) | 12 (46%) | Most common adverse event (AE) was headaches |
| Vogelmeier et al.24 | 75 (12%) | 211 (34%) | Two cases of pneumonia; most common AE was nasopharyngitis | |
| Kuna et al.23 | 24 (7.6%) | 53 (17%) | – | |
| Gunawardena et al.19 | 1 (5%) | 2 (11%) | Most common AE was headache | |
| Stockley et al.17 | 2 (9%) | 15 (68%) | Seven cases of headache; four cases of nasopharyngitis | |
| Nordenmark et al.26 | 4 (16%) | 14 (56%) | – | |
| BAY 85-8501 | Watz et al.27 | 10 (22%) | 31 (69%) | Ten cases of gastrointestinal effects like diarrhea |
| POL6014 | Barth et al.29 | 0 (0%) | 5 (27%) | One subject was on 80 mg; 2 were on 160 mg and 320 mg |
| AAT | Martin et al.21 | 0 (0%) | 0 (0%) | Two cases of antibodies developed against the treatment but they did not have adverse events |
| Griese et al.22 | 20 (27%) | 15 (28%) | – | |
| Stolk et al.28 | 34 (40%) | 49 (57.5%) | Most common AEs were dyspnea and coughs. | |
| Alpha1-PI | Häussermann et al.20 | 0 (0%) | 7 (33%) | – |
Discussion
To the best of my knowledge, this systematic review is the first to examine the impact of neutrophil elastase inhibitors on chronic lung illnesses. Consequently, the current review offers a comprehensive summary and crucial advice for future research on NE inhibitors for chronic lung disease patients.
Summary of Main Results
Twelve studies involving a total sample of 1993 subjects with chronic lung conditions (n=186 for CF, n=1675 for COPD, n=132 for bronchiectasis) were included. The current findings describe seventeen years of neutrophil elastase inhibitor treatment for patients with chronic lung disease. Reviewed studies applied neutrophil elastase inhibitors to (1) test the tolerability and safety of the drug, (2) reduce NE levels, or (3) improve lung function and quality of life.
Nine of the studies presented an excellent or good level of evidence because they were controlled, randomized clinical trials with satisfactory sample sizes of more than 30 subjects.17,19,21,23-28 Four of these studies reported statistically significant positive effects of NE inhibitors associated with inflammatory biomarkers17,21,25 , FEV117, and NE activity.27 Involving either CF or bronchiectasis patients, these four studies included AZD9668, BAY 85-8501, and AAT treatments.
Out of the nine studies with excellent levels of evidence, five reported a general positive yet statistically insignificant effect of the treatment associated with NE activity,17,21,25 inflammatory biomarkers,21,25 FEV1,28 and lung function.26 These studies tested the NE inhibitor AZD9668 on individuals with bronchiectasis,17 CF,25 or COPD24,26 as well as the NE inhibitor AAT on patients with CF.21 However, these results were not statistically significant, so the associated results should be interpreted with caution.
The implications of these findings suggest the need for further investigation into refining treatment protocols. Despite the absence of statistically significant results in certain studies, the overall trend toward improvements in NE activity, inflammatory biomarkers, and lung function highlights the potential clinical relevance of these inhibitors in managing chronic lung diseases.
Heterogeneity
This review found a great level of heterogeneity. Overall, studies presented a wide range of protocols. It remains unclear which treatment, frequency, and duration of treatment are essential for optimal clinical benefits for chronic lung patients. In addition, studies measured diverse outcomes. Variations in chronic lung disease type, degree of sickness, and patients’ ages were frequently observed across the study samples. This heterogeneity stems from the different mechanisms and features of chronic lung illnesses. For example, trials involving CF patients typically possess younger sample group than COPD studies because the former condition is diagnosed in childhood and the latter beyond age 50. Because all studies reviewed applied NE inhibitors in subjects with one of three chronic lung diseases (CF, COPD, and bronchiectasis), conclusions drawn in this review can only be applied to individuals with these conditions.
Quality of Evidence and Limitations
The overall lack of statistical power observed in the current body of literature prevents firm conclusions about NE inhibitors’ efficacy for chronic lung disease patients, despite the various types of NE inhibitors being developed. Studies that were not randomized, controlled, nor contained good sample size were regarded as having a high risk of bias. The current review contains nine studies with low risk of bias.
Out of those nine studies, the statistically significant positive results were mainly a reduction of inflammatory biomarkers, which has been associated with improvements in clinical symptoms among patients with conditions like CF,30 COPD,31 and bronchiectasis.32 However, people with the same lung condition can exhibit variations in correlation between biomarkers and lung function,30 so these individual differences make it challenging to establish a consistent relationship.
Implications and Future Research
Based on this review, it is recommended that patients undergo a longer treatment period at a higher dosage. Some papers pointed out that the lack of statistically significant data could potentially stem from a short duration of treatment as well as a low dosage.22,27 Studies averaged around four weeks of treatment. Participants in Stolk et al.28 underwent the longest duration of treatment for 50 weeks of AAT and saw a clinical improvement in lung function. However, this outcome was statistically insignificant which may be a potential focus for future studies.
Based on the current literature, more studies should focus on NE inhibitors’ ability to inhibit NE activity. While animal studies have found significant decreases in NE activity13,14 as expected, only two studies found significant decrease of NE activity following treatment. NE inhibitors aim to improve lung function by inhibiting and reducing NE. Therefore, the lack of significant improvement in the human lung might stem from the lack of significant inhibition of NE by the inhibitors. Future studies on NE inhibitors should focus on resolving this issue.
Limitations of the Systematic Review
Because this review had three treatments with only one clinical study conducted (BAY 85-8501, POL6014, Alpha1-PI), definitive conclusions could not be made about the efficacy of those NE inhibitor treatments. The limited number of studies for each treatment restricts drawing effective conclusions, and a more extensive body of research is needed to establish treatment efficacy. Furthermore, the time constraint given to write this review hindered additional analysis of the results and collection of data. Due to this limitation, the review may not include all potentially relevant studies, and data extraction may have been limited in scope.
Conclusion
Overall, the current trials with NE inhibitors on patients with chronic lung conditions have found little to no clinical improvements regarding lung function. While a significant reduction of sputum inflammatory biomarkers was observed with the NE inhibitors AZD9668 and AAT, this finding should be taken with caution as most of these studies reported no other significant improvements in the lung. Furthermore, many of the NE inhibitors in trials did not significantly inhibit NE in patients. Future studies should investigate further into NE inhibitors’ ability to inhibit NE in human subjects before exploring its clinical benefits on patients.
Notes
- FEV1 refers to the maximum amount of air a person can forcefully exhale in one second after taking a deep breath. A high FEV1 indicates that the individual can expel a large volume of air from their lungs in one second, which typically suggests good lung capacity. Low FEV1 suggests reduced lung function and respiratory issues. Comparing the FEV1 pre and post treatment, researchers evaluated how effective a medication is in improving lung function. ↩︎
References
- Naehrig S, Chao CM, Naehrlich L. Cystic fibrosis. Deutsches Ärzteblatt International. 2017:114(33-34):564-574.
- Ruvuna L, Sood A. Epidemiology of chronic obstructive pulmonary disease. Clinics in Chest Medicine. 2020:41(3):315–327.
- Butler MW, Keane MP. Bronchitis, bronchiectasis. Infectious Diseases. 2017:1:243-250.
- Soriano J, Kendrick P, Paulson K, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020:8(6):585–596.
- Dechecchi MC, Tamanini A, Cabrini G. Molecular basis of cystic fibrosis: From bench to bedside. Ann Transl Med. 2018:6(17):334–334.
- MacNee W. Pathology, pathogenesis, and pathophysiology. BMJ. 2006:332(7551):1202–1204.
- Chalmers JD, Elborn S, Greene CM. Basic, translational and clinical aspects of bronchiectasis in adults. Eur Respir Rev. 2023:32(168):230015.
- Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. 2021:54(7):1377–1391.
- Laval J, Ralhan A, Hartl D. Neutrophils in cystic fibrosis. Biol Chem. 2016:397(6):485–496.
- Voynow JA, Shinbashi M. Neutrophil elastase and chronic lung disease. Biomolecules. 2021:11(8):1065.
- Yamakoshi Y. Dental and oral biology, biochemistry. Reference Module in Biomedical Sciences. 2014.
- Domon H, Nagai K, Maekawa T, et al. Neutrophil elastase subverts the immune response by cleaving toll-like receptors and cytokines in pneumococcal pneumonia. Front Immunol. 2018:9:1-11.
- Polverino E, Rosales-Mayor E, Dale G, et al. The role of neutrophil elastase inhibitors in lung diseases. Chest. 2017:152(2):249–262.
- Fantone K, Crippen C, Nothaft H, et al. 255 A bacterial serine protease inhibitor, Ecotin inhibits neutrophil elastase in cystic fibrosis airway samples. J Clin Transl Sci. 2022:6(Suppl 1):42.
- Twigg MS, Brockbank S, Lowry P, Fitzgerald P, Taggart C, Weldon S. The role of serine proteases and antiproteases in the cystic fibrosis lung. Mediators of Inflamm. 2015:2015:1–10.
- Sakashita A, Nishimura Y, Nishiuma T, et al. Neutrophil elastase inhibitor (sivelestat) attenuates subsequent ventilator-induced lung injury in mice. Eur J Pharmacol. 2007:571(1):62–71.
- Stockley R, De Soyza A, Gunawardena K, et al. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir Med. 2013:107(4):524-533.
- Elborn JS, Perrett J, Forsman-Semb K, Marks-Konczalik J, Gunawardena K, Entwistile N. Efficacy, safety and effect on biomarkers of azd9668 in cystic fibrosis. Eur Respir J. 2012:40(4):969-976.
- Gunawardena KA, Gullstrand H, Perrett J. Pharmacokinetics and safety of AZD9668, an oral neutrophil elastase inhibitor, in healthy volunteers and patients with COPD. Int J Clin Pharmacol Ther. 2013:51(4):288–304.
- Häussermann S, Winnips C, Edelman J, et al. Lung deposition of alpha1-proteinase inhibitor (human) (a1-pi[h]) inhalation solution using two inhalation modes of the I-Neb Adaptive Aerosol Delivery (AAD) system in healthy subjects and subjects with cystic fibrosis. J Aerosol Med Pulm Drug Deliv. 2016:29(3):242-250.
- Martin SL, Downey D, Bilton D, et al. Safety and efficacy of recombinant alpha1-antitrypsin therapy in cystic fibrosis. Pediatr Pulmonol. 2006:41(2):177-183.
- Griese M, Latzin P, Kappler M, et al. 1-antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J. 2007:29(2):240-250.
- Kuna P, Jenkins M, O’Brien CD, Fahy WA. AZD9668, a neutrophil elastase inhibitor, plus ongoing budesonide/formoterol in patients with COPD. Respir Med. 2012:106(4):531–539.
- Vogelmeier C, Aquino TO, O’Brien CD, Perrett J, Gunawardena KA. A randomised, placebo-controlled, dose-finding study of AZD9668, an oral inhibitor of neutrophil elastase, in patients with chronic obstructive pulmonary disease treated with tiotropium. COPD 2012:9(2):111–120.
- Elborn JS, Perrett J, Forsman-Semb K, Marks-Konczalik J, Gunawardena K, Entwistle N. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur Respir J. 2012:40(4):969-976.
- Nordenmark LH, Taylor R, Jorup C. Feasibility of computed tomography in a multicenter COPD trial: A study of the effect of AZD9668 on structural airway changes. Adv Ther. 2015:32(6):548–566.
- Watz H, Nagelschmitz J, Kirsten A, et al. Safety and efficacy of the human neutrophil elastase inhibitor bay 85-8501 for the treatment of non-cystic fibrosis bronchiectasis: A randomized controlled trial. Pulm Pharmacol Ther. 2019:56:86-93.
- Stolk J, Tov N, Chapman K, et al. Efficacy and safety of inhaled α1-antitrypsin in patients with severe α1-antitrypsin deficiency and frequent exacerbations of COPD. Eur Respir J. 2019:54(5):1900673.
- Barth P, Brujinzeel P, Wach A, et al. Single dose escalation studies with inhaled POL6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J Cyst Fibros. 2020:19(2):299-304.
- Levy H, Kalish L, Huntington I, et al. Inflammatory markers of lung disease in adult patients with cystic fibrosis. Pediatr Pulmonol. 2007:42(3):256-262.
- Şahin F, Koşar AF, Aslan AF, Yiğitbaş B, Uslu B. Serum biomarkers in patients with stable and acute exacerbation of chronic obstructive pulmonary disease: A comparative study. J Med Biochem. 2019:28(4):503-511.
- Lee S, Jeong J, Heo M, et al. Serum fibrinogen as a biomarker for disease severity and exacerbation in patients with non-cystic fibrosis bronchiectasis. J Clin Med. 2022:11(14):3948.
Supplementary Figures and Tables
Extended Data Table 1. Eligibility Criteria and Outcomes Measured
| Inclusion criteria | Exclusion criteria | |
| Chronic lung conditions | Individuals with cystic fibrosis, chronic obstructive pulmonary disease, or bronchiectasis | Individuals not having any of the three conditions |
| Intervention | Any type of neutrophil elastase inhibitor aiming to improve lung function | Interventions such as surgeries, lung transplants, and other types of inhibitors |
| Study design | Randomized clinical trials | Reviews, conference abstracts, research protocols, ongoing studies, case studies, opinions, and comments |
| Primary outcome | Lung function improvements using standardized and non-standardized assessments | NA |
| Secondary outcomes | Safety of the neutrophil elastase inhibitor using adverse events and side effects | NA |
Extended Data Figure 1. Flow diagram for screening and search procedure
Extended Data Table 2. General descriptive characteristics of included studies
| Study | Time of publication | Study site |
| Martin et al.21 | 2006 | UK |
| Griese et al.22 | 2006 | Germany |
| Kuna et al.23 | 2012 | Bulgaria, Czech Republic, Hungary, Poland, Romania, Slovakia, |
| Vogelmeier et al.24 | 2012 | Australia, Canada, Germany, Japan, Philippines, Poland, Republic of Korea, Russian Federation, Slovakia, Taiwan, Ukraine, USA |
| Elborn et al.25 | 2012 | Denmark, Germany, Poland, Russian Federation, Sweden, UK |
| Gunawardena et al.19 | 2013 | Germany |
| Stockley et al.17 | 2013 | Canada, UK |
| Nordenmark et al.26 | 2015 | Canada, Denmark, Netherlands, Romania, Ukraine |
| Häussermann et al.20 | 2016 | Germany, Switzerland, USA |
| Watz et al.27 | 2019 | France, Germany, Italy, Spain, UK |
| Stolk et al.28 | 2019 | Canada, Denmark, Germany, Ireland, Netherlands, Sweden, UK |
| Barth et al.29 | 2020 | Germany |
Extended Data Table 3. Characteristics of patients with chronic lung disease included in the studies
| Studies | Sample of patients | Condition | Age (years mean) | Sex (male/female) | Race |
| Elborn et al.25 | 56 | CF | 28 | 53/1 | 56 Caucasian |
| Barth et al.29 | 24 | CF | 30 | 23/1 | 24 Caucasian |
| Martin et al.21 | 39 | CF | 28 | 32/7 | 39 Caucasian |
| Griese et al.22 | 52 | CF | 25 | 26/26 | 52 Caucasian |
| Häussermann et al.20 | 15 | CF | 32.9 | 12/3 | – |
| Vogelmeier et al.24 | 838 | COPD | 62 | – | – |
| Kuna et al.23 | 615 | COPD | 61 | 454/161 | 610 Caucasian; 5 Other |
| Gunawardena et al.19 | 18 | COPD | 55.5 | 13/5 | 18 Caucasian |
| Nordenmark et al.26 | 52 | COPD | 65 | 36/16 | 36 Caucasian |
| Stolk et al.28 | 168 | COPD | 55 | 100/68 | – |
| Watz et al.27 | 94 | Bronchiectasis | 66.3 | 53/41 | 91 Caucasian; 1 Black; 2 Asian |
| Stockley et al.17 | 38 | Bronchiectasis | 62 | 18/20 | 33 Caucasian; 1 Black; 3 Asian; 1 Other |
Extended Data Table 4. Primary outcomes that are not statistically significant P>.05
| Treatment | Studies | Neutrophil count | NE activity | inflammatory biomarkers | FEV1 | Lung Function |
| AZD9668 | Elborn et al.25 | X | O | O | X | X |
| Vogelmeier et al.24 | – | – | X | X | X | |
| Kuna et al.23 | – | – | X | X | X | |
| Gunawardena et al.19 | – | X | – | – | – | |
| Stockley et al.17 | X | O | O | X | X | |
| Nordenmark et al.26 | – | – | – | X | O | |
| BAY 85-8501 | Watz et al.27 | X | X | X | X | X |
| POL6014 | Barth et al.29 | – | O | – | X | – |
| AAT | Martin et al.21 | – | O | O | X | X |
| Griese et al.22 | X | X | O | X | X | |
| Stolk et al.28 | – | – | – | O | X | |
| Alpha1-PI | Häussermann et al.20 | – | – | – | X | X |
Acknowledgements: I am immensely grateful to Dr. Holly Gallagher for her unwavering support, her thorough feedback, and her encouragement throughout the writing process.
Citation Style: AMA